SYMBOLS GLOSSARY
| Symbol | Title | Reference | Description |
|---|---|---|---|
 |
Fragile, handle with care |
ISO 15223-1, 5.3.1
ISO 7000 - 0621
|
Indicates a medical device that can be broken or damaged if not handled carefully |
 |
This way up |
ISO 7000 - 0623
|
To indicate correct upright position of the transport package. |
 |
Keep away from sunlight |
ISO 15223-1, 5.3.2
ISO 7000 - 0624
|
Indicates a medical device that needs protection from light sources. NOTE: This symbol can also mean “Keep away from heat”. |
 |
Keep dry |
ISO 15223-1, 5.3.4
ISO 7000 - 0626
|
Indicates a medical device that needs to be protected from moisture. |
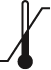 |
Temperature limit |
ISO 15223-1, 5.3.7
ISO 7000 - 0632
|
To indicate the maximum and minimum temperature limits at which the item shall be stored, transported or used |
 |
Consult instructions for use |
ISO 15223-1, 5.4.3
ISO 7000 - 1641
|
Indicates the need for the user to consult the instructions for use. |
 |
Do not stack |
ISO 7000 - 2402
|
Stacking of the distribution packages is not allowed and no load shall be placed on the distribution packages. |
 |
Authorized representative in the European Community |
ISO 15223-1, 5.1.2
|
Indicates the authorized representative in the European Community / European Union |
 |
Lot number |
ISO 15223-1, 5.1.5
ISO 7000 - 2492
|
Indicates the manufacturer’s batch code so that the batch or lot can be identified. |
 |
Date of manufacture |
ISO 15223-1, 5.1.3
ISO 7000 - 2497
|
Indicates the date when the medical device was manufactured. |
 |
Manufacturer |
ISO 15223-1, 5.1.1
ISO 7000 - 3082
|
Indicates the legal medical device manufacturer. |
 |
Country of manufacture |
ISO 15223-1, 5.1.11
ISO 7000 - 6049
|
To identify the country of manufacture of products. |
 |
Importer |
ISO 15223-1, 5.1.8
ISO 7000 - 3725
|
Indicates the entity importing the medical device into the locale. |
 |
Catalog number |
ISO 15223-1, 5.1.6
ISO 7000 - 2493
|
Indicates the manufacturer’s catalogue number so that the medical device can be identified. |
 |
Batch code |
ISO 15223-1, 5.1.5
ISO 7000 - 2492
|
Indicates the manufacturer’s batch code so that the batch or lot can be identified. |
 |
Serial number |
ISO 15223-1, 5.1.7
ISO 7000 - 2498
|
Indicates the manufacturer’s serial number so that a specific medical device can be identified. |
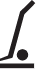 |
Use hand truck |
N/A
|
To indicate that a hand truck or dolly should be used. |
 |
General symbol for recovery/recyclable |
ISO 7000 - 1135
|
To indicate that the marked item or its material is part of a recovery or recycling process. |
 |
Do not clamp as indicated |
ISO 7000 – 2404
|
To indicate the sides of a transport package which shall not be clamped when handling the package by mechanized means. |
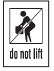 |
Do Not Lift |
N/A
|
To indicate a heavy object; do not lift |
 |
Stacking limit by number |
ISO 7000 - 2403
|
To indicate that the items shall not be vertically stacked beyond the specified number, either because of the nature of the transport packaging or because of the nature of the items themselves. |
 |
Exclamation Mark |
Globally Harmonized System (GHS) Pictogram
|
To indicate that there are health or physical hazard/s associated with a chemical product |
 |
Use Two Person Lift |
N/A
|
To indicate that two persons must lift the object. |
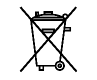 |
WEEE; waste electrical and electronic equipment |
IEC 60417 - 6414
|
To indicate that separate collection for waste electric and electronic equipment (WEEE) is required. |
 |
Caution, risk of electric shock |
IEC 60417 - 6042
|
To identify equipment, for example, the welding power source, that has risk of electric shock. |
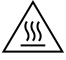 |
Caution, hot surface |
IEC 60417 - 5041
|
To indicate that the marked item can be hot and should not be touched without taking care. |
 |
Caution |
ISO 7000 – 0434B
|
To indicate that caution is necessary when operating the device or control close to where the symbol is placed. To indicate that the current situation needs operator awareness or operator action in order to avoid undesirable consequences. On the health app quality label: to indicate that the health app requires approval from a health professional for use. |
 |
Medical device |
ISO 15223- 1, 5.7.7
|
Indicates the item is a medical device |
 |
Unique Device Identifier |
ISO15223-1, 5.7.10
|
Indicates a carrier that contains Unique Device Identifier information |
 |
Use by date |
ISO 15223-1, 5.1.4;
ISO 7000 - 2607
|
To indicate that the device should not be used after the date accompanying the symbol, for example on a medical device or its packaging. |
 |
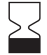
Distilled water only |
N/A
|
Distilled water only |
 |
CE mark |
MDR 2017/745; Art 20
|
European Union certification mark |
 |
Fuse |
IEC 60417 - 5016
|
Fuse - Type and rating are marked next to the symbol |
 |
No defined expiry |
N/A
|
The product has an indefinite expiry. |

